
COVID-19 – Indonesia issues Sinovac vaccine use permit for 6-11 year children
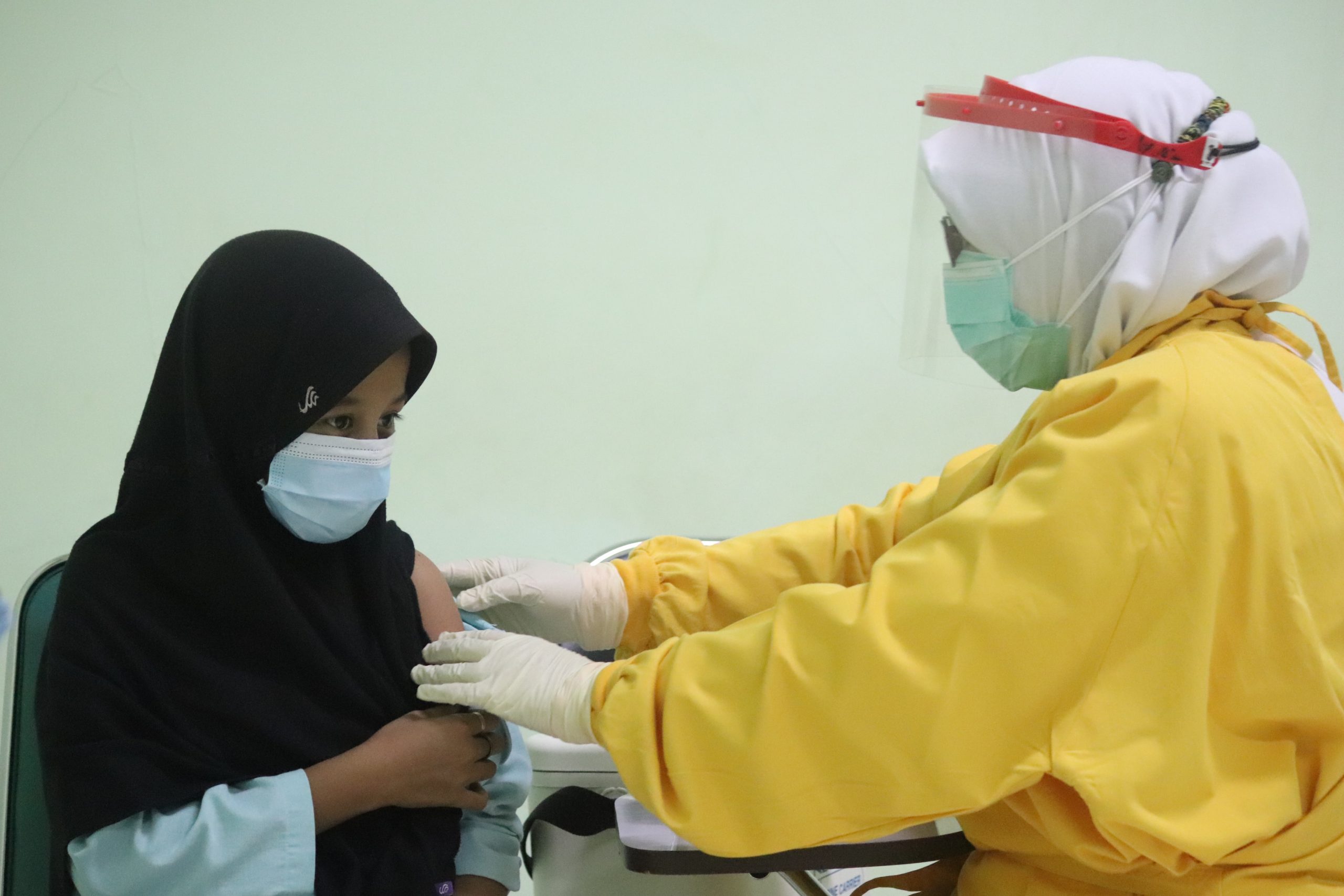
Illustration. (Mufid Majnun on Unsplash)
Bagikan

Komentar
Berita Terkait

Vice President delivers Indonesia’s Vision 2045 when chairing a UN session
Indonesia
•
26 Sep 2019

Indonesia calls on OIC to unite, be at forefront in resolving crisis in Gaza
Indonesia
•
13 Nov 2023

Taiwan clarifies case of visa fraud and forgery against Indonesian migrant workers
Indonesia
•
30 Mar 2021

Embassy finds 3 Indonesians missing in Saudi for decades
Indonesia
•
09 Sep 2019


Berita Terbaru

Ramadan 1447H – Bogor ’s speaker says Muslim students are Indonesia’s outstanding generation as they study the Quran
Indonesia
•
11 Mar 2026

Ramadan 1447H - UAE distributes 1.59 billion rupiahs in aid for fast breaking of Muslims at Istiqlal Mosque in Jakarta
Indonesia
•
09 Mar 2026

Indonesia increasingly vulnerable to tropical cyclones
Indonesia
•
08 Mar 2026

Condition in Gaza causes sharp turn in history
Indonesia
•
07 Mar 2026
