COVID-19 – Indonesia issues Sinovac vaccine use permit for 6-11 year children
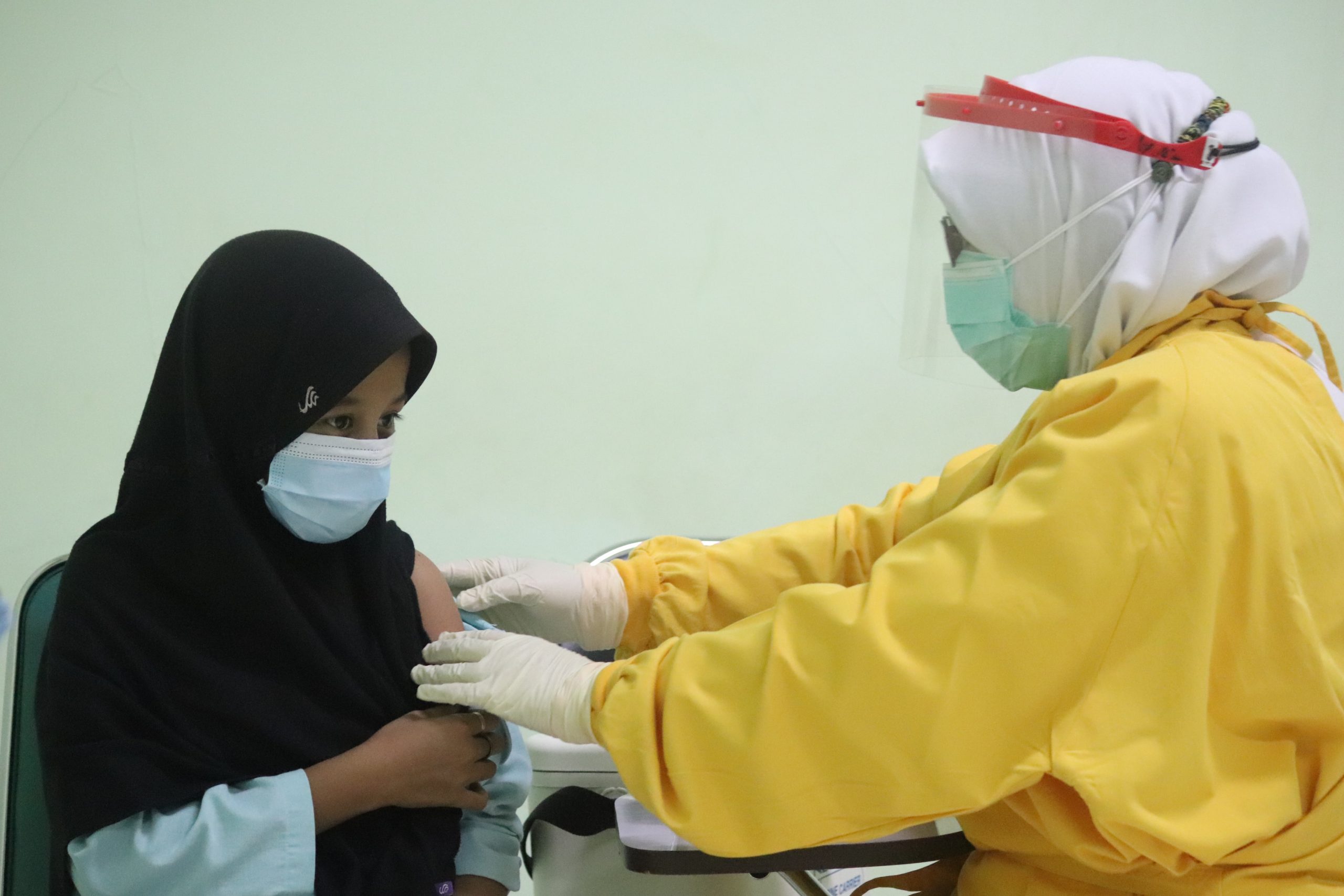
Jakarta (Indonesia Window) – Indonesia’s National Agency of Drug and Food Control has issued a permit to use Sinovac vaccine for children aged 6 to 11 years old, referring to results of the safety and immunity assessment against COVID-19.
“The clinical trials in children are more about safety and immunogenicity aspects. The safety aspect shows that this (vaccine) is safe for children aged 6 to 11 years old,” said the agency’s head, Penny Lukito, at a press conference here on Monday (Nov. 1).
Penny added that the issuance of permit for the use of COVID-19 vaccines for children was urgent considering that currently schools have started to implement limited offline classes.
The report on clinical trial results stated that the side effects coming up from vaccination in children are similar to those aged 11 to 17 years old.
The permit to use the Sinovac vaccine for children aged 11 to 17 years was previously issued and declared safe for use.
The report also shows that the immunogenicity or ability of the vaccine to generate children body’s immune response is greater than that found in adults, with 96.15 percent in the former compared to 89.04 percent in the latter.
Penny pointed out that the Sinovac vaccine is the first vaccine registered in the agency for children aged 6-11 years old.
She expected that there would be other COVID-19 vaccines registered by the agency soon, and have qualifications to be used for children aged 6-11 years old.
Reporting by Indonesia Window
.jpg)